This is my most precious instrument. It contains 4 Weston saturated standard cells. These units where the ultimate Voltage standards for many decades. Labs like NIST used them as the highest level for calibration.
The first generation was the Daniel cell, invented in 1836. It’s value was 1,08V. It was not very stable and did not last a long time. But electronics was still in the experimental stage and the multimeter not invented yet. In 1872 it was replaced by the Clarkcell. This was the first commercially made cell. The tempco ( 0.00115 V/°C) was a bit high for a standard and the glass often broke at the place the platinum wires entered the glass. Lord Rayleigh improved it in 1894. It became known as the Board of Trade Cell. It was 1,434V at 15°C. He came up with a formula to calculate the voltage at other temperatures. To bad they found out it was 0,1% off. The next generation was the Reichsanstalt, made by Kahle. The EMF was 1,4328 international volts according to the Laws Electrical Measurements uit 1917
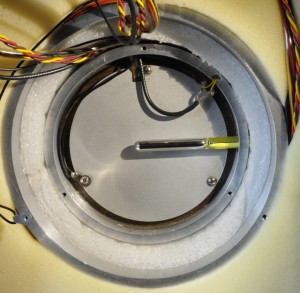
Edward Weston (1850-1936) filed a patent in 1891 for his cell. He replaced cadmium by zinc and cadmium sulfate by zinc sulfate electrolyte. This cell was stable and reproducible with no glass problems. The Weston Standard Cell is a H shaped glass construction. In the bottom of each tube is a platinum wire. It is coil shaped to prevent damage caused by movements. One cell contains mercury covert with mercury sulfate. This is the positive electrode. The negative tube contains cadmium mercury amalgam. Above this, a saturated cell has a layer of cadmium sulfate crystal. Non saturated cells have a top-layer of cadmium sulfate. This makes it possible to transport them and use other shapes. The downside is a short lifespan.
My unit contains 4 cells. Three is the minimum. One is never used for calibrating other instruments, it is only a reference for it’s own calibration. The other 2 have a known value so the difference is known too. If you are in doubt you measure both, this way you know if it’s the meter or the cells that are off value. My cells are made in 1973, the cabinet in 1974. The last calibration was in 1987. After that it stayed in the lab with a broken heater. So an almost perfect situation. I have the complete calibration history. In the 14 years of use the best cell was decreased 600 nV. Cell 3 is now 1.018,133,5V. The other three have the same difference in voltage as in 1987. In theory the worst case value has to be between 1.018,120,2 1.018,150,7 V. But several measurements show a value close to 1.018,133,5V
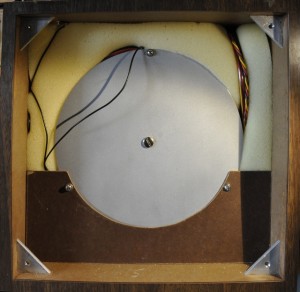
One important thing for these cell is the temperature. That must be mega stable. And that is why most of the cabinet is occupied by the oven. Transporting the cabinet is very tricky. After some movements the cells need 2 weeks to stabilize. The cabinet needs to be kept in the same position. Turning it up side down or shaking it to much can damage the cells. Mine are transported from the old lab to my lab with great care by a friend.
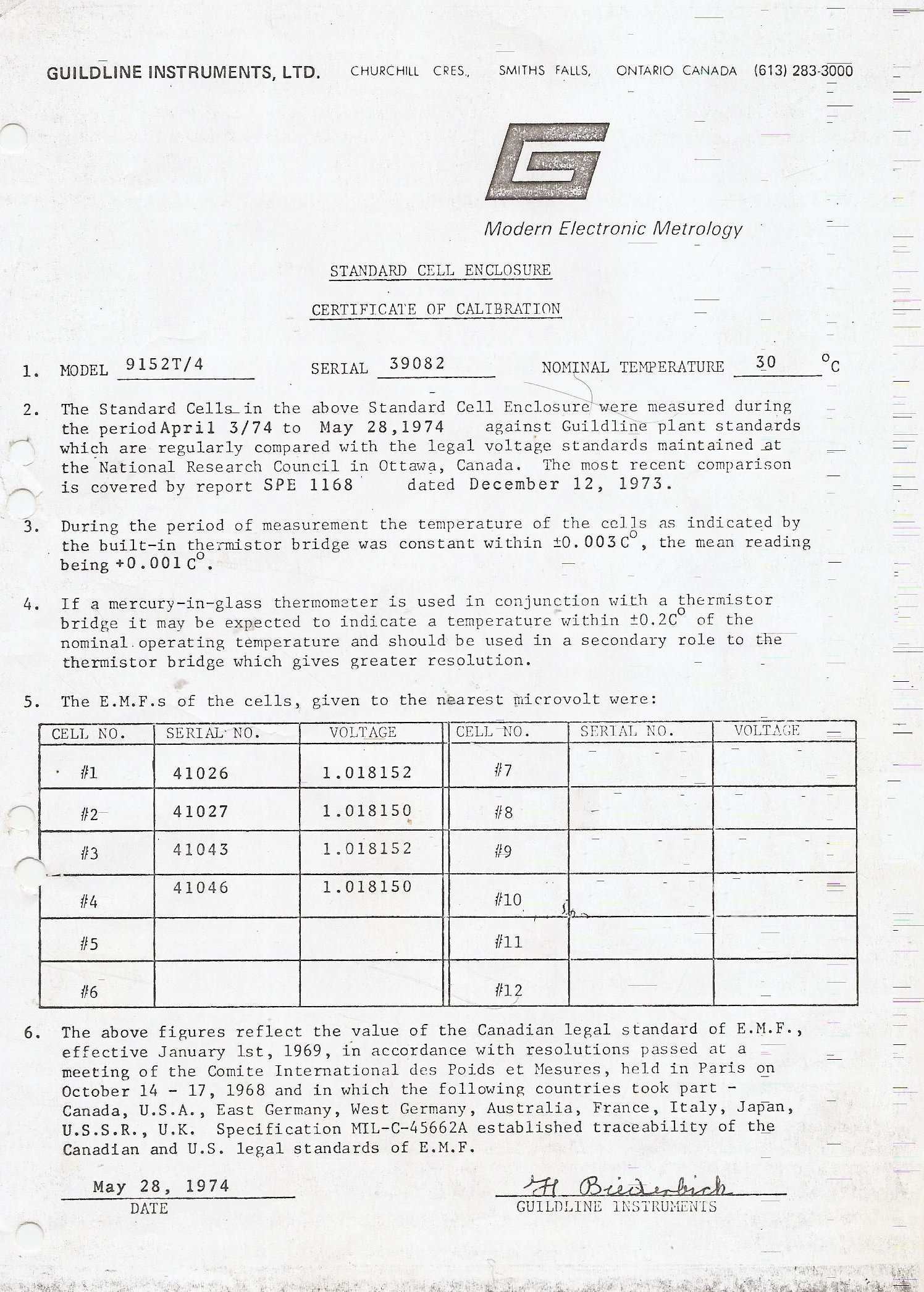
The most outer shell of the oven is surrounded by polystirene foam. The tempcontroller had a fault. All electrolytic caps failed. A trace was broken due to disintegrated solder.
Cells need to be handeled with the greatest care. You can not measure them direct. That will drain to much current. They are used with a null detector and an electronic voltage standard. Today some meterology grade instruments are >10 Gohm and that is a safe way to measure them. The null-detector method is a very nice way to make an accurate compare between 2 sources. If they are equal there is no voltage difference and the meter shows zero. And zero is very easy to calibrate. This way the nullmeter specs do not matter as log as no signal shows 0.
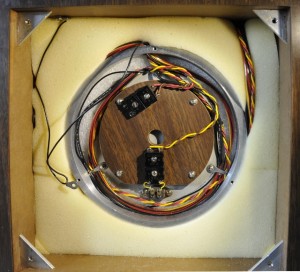
The second shell. This holds the heater. There are several protection devices. The oven has to be within 0.003 degrees at 30 degrees over 24 hours.